Literature
- Immunity literature
- Skin-care literature
- Anti-alcohol literature
- Detoxification literature
- GI health literature
- Cholesterol health literature
- back
Contact us
Zhejiang Gold Kropn Bio-technology Co.,Ltd.
Tal:0570-8788056
Fax:0570-8788381
E-mail:zjgykp@163.com
Address:No.2,Donggang 4 Road,Donggang Economic Development Zone,Quzhou City,Zhejiang Province
- THE ROLE OF MODIFIED CITRUS PECTIN AS AN EFFECTIVE CHELATOR OF LEAD IN CHILDREN HOSPITALIZED WITH TOXIC LEAD LEVELS
- date: 2019/4/12 visits:3331
THE ROLE OF MODIFIED CITRUS PECTIN AS AN EFFECTIVE CHELATOR OF LEAD IN CHILDREN HOSPITALIZED WITH TOXIC LEAD LEVELS
Zheng Yan Zhao, MD; Li Liang, MD; Xiaoqing Fan, MD; Zhonghua Yu, PhD;
Arland T. Hotchkiss, PhD; Barry J. Wilk, MA; Isaac Eliaz, MD, MS, LAc
Context • Lead toxicity is an ongoing concern worldwide, and chil- dren, the most vulnerable to the long-lasting effects of lead expo- sure, are in urgent need of a safe and effective heavy metal chelating agent to overcome the heavy metals and lead exposure challenges they face day to day.
Objective • This clinical study was performed to determine if the oral administration of modified citrus pectin (MCP) is effective at lowering lead toxicity in the blood of children between the ages of 5 and 12 years.
Method • Hospitalized children with a blood serum level greater than 20 µg/dL, as measured by graphite furnace atomic absorption spectrometry (GFAAS), who had not received any form of chelating and/or detoxification medication for 3 months prior were given 15g of MCP (PectaSol) in 3 divided dosages a day. Blood serum and 24-hour urine excretion collection GFAAS analysis were performed on day 0, day 14, day 21, and day 28.
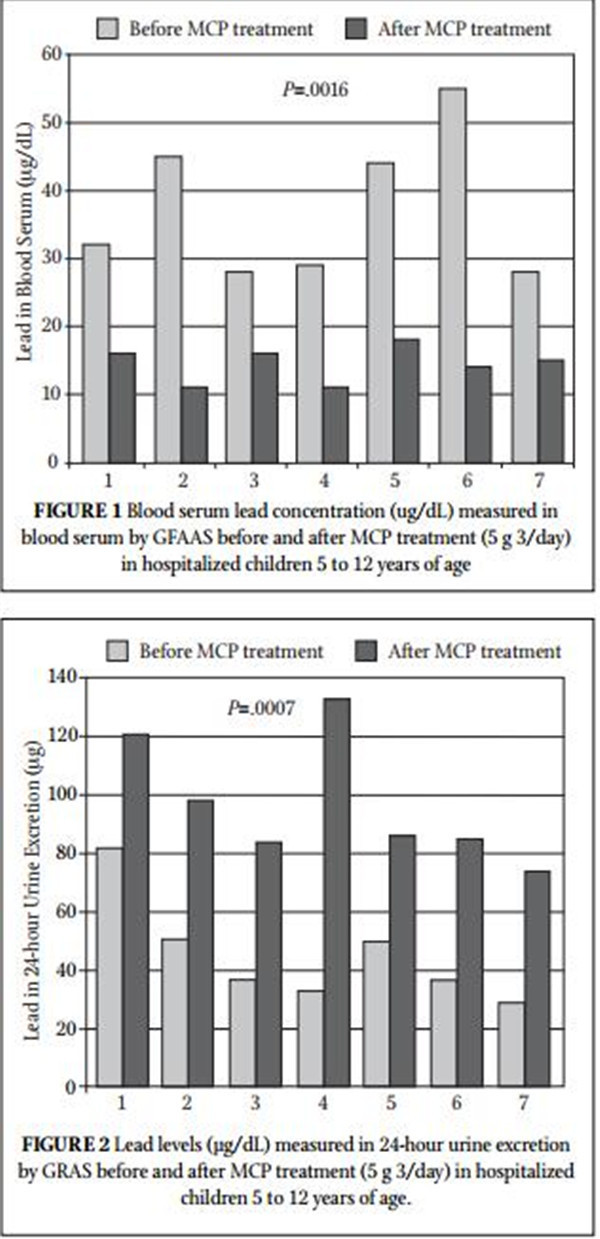
Result • This study showed a dramatic decrease in blood serum levels of lead (P=.0016; 161% average change) and a dramatic increase in 24-hour urine collection (P=.0007; 132% average change).
Conclusion • The need for a gentle, safe heavy metal–chelating agent, especially for children with high environmental chronic expo- sure, is great. The dramatic results and no observed adverse effects in this pilot study along with previous reports of the safe and effec- tive use of MCP in adults indicate that MCP could be such an agent. Further studies to confirm its benefits are justified. (Altern Ther Health Med. 2008;14(4):34-38.)Zheng Yan Zhao, MD, and Li Liang, MD, are professors in the Department of Medicine, Children’s Hospital, Zhejiang University School of Medicine, Hangzhou, Republic of China.
Xiaoqing Fan, MD, is president and chief medical officer and
Zhonghua Yu, PhD, is vice president, research, at Centrax International, Inc, San Francisco, California. Arland T. Hotchkiss, PhD, is lead scientist at the Eastern Regional Research Center, Agricultural Research Service, US Department of Agriculture, Wyndmoor, Pennsylvania. Barry J. Wilk, MA, is director of Research and Development at EcoNugenics, Inc, Santa Rosa, California. Isaac Eliaz, MD,
MS, LAc, is medical director of Amitabha Medical Clinic and Healing Center, Sebastopol, California.Lead is a ubiquitous metal. It is in our environment, with some areas in the world having higher levels than oth- ers. Lead toxicity is one of the most, if not the most, important chronic environmental illness affecting chil- dren today. Despite efforts to control it, serious cases oflead poisoning still appear throughout the world, especially in developing countries.
In the very young, virtually no system in the body is immune to the effects of lead toxicity. Lead disturbs multiple enzyme sys- tems. The part of the developing body of greatest concern is the brain. Damaging actions at this critical time in development are likely to have long-lasting effects. Effects of early lead toxicity on the brain appear to continue into the teenage years and later periods of life. Significant insult to the brain occurs at very low levels of lead toxicity, and medical intervention with traditional chelation fails to reverse such effects.1,2 Lead poisoning affects the brain in many ways, leading to delayed or reversed development, permanent learning disabilities, seizures, coma, and even death.3-5 In the United States, lead poisoning is the most common environmental illness in children. The occurrence of lead poisoning is infl uenced to some degree by economic status, the population size of a given communi- ty, race, and the age of the home in which a person lives, but lead poisoning can occur in any community; only the frequency varies.
Lead poisoning has been reported in almost every country on earth. A report from 1996 found that Chinese children residing in industrial and busy traffic areas had average blood lead levels
(BPb) of 21.8 to 67.9 µg/dL. The percentages of BPb values above 10 µg/dL, which is the threshold for lead poisoning in children, ranged from 64.9% to 99.5%. Even approximately 50% of “unex- posed” children had BPb values above 10 µg/dL.6 The correlation between low-level lead exposure and deficits in IQ, neurobehavior- al development, and physical growth is remarkably consistent without exception.7
Lead poisoning in adults usually occurs through occupational exposure. A recent article presented a summary of lead exposure lev- els and lead poisoning at workplaces in China reported in the Chinese medical literature between 1990 and 2005.8 The authors found that approximately 53.7% of the averages reported in the papers were above the national maximum occupational exposure levels.
Existing chelating agents have significant adverse side effects associated with them, can bind to essential minerals in the body, require medical monitoring because they are often administered intravenously, and often are not safe for use in children. Modified citrus pectin (MCP) is a purifi ed component of standard citrus pectin, which is officially recognized as generally regarded as safe
(GRAS). MCP is thought to have the same level of safety as unmod- ified pectin, which is an established food additive.9
MCP is a dietary supplement derived from the inner white pulp of citrus fruit peels. Citrus pectin is a complex polysaccharide that is a soluble fiber. D-galacturonic acid is the principal monosac- charide in pectin, but neutral sugars are also present. The D-galacturonic acid residues are linked together by alpha-1, 4 glyco- sidic linkages. Unmodified pectin is a non-digestible polysaccharide in long polymers of cross-linked chains. The MCP used in this study is composed of citrus pectin that has been broken down into short- er chain molecules and reduced side chain structure using enzymat- ic and pH modification. The lower molecular weight may facilitate MCP absorption into the bloodstream, and the reduced esterifica- tion enhances the ability of the pectin molecule to bind to cations. MCP is known for its effects on inhibiting cancer metastasis10 and reducing tumor growth11 and progression12 but also has beneficial effects on cholesterol reduction13 and may stimulate the immune system.14 MCP’s ability to bind toxic heavy metals and excrete them while not disturbing the essential minerals in healthy humans has been demonstrated.15 MCP was demonstrated to have optimal structure for chelation of heavy metals. It consists of approximately 10% rhamnoglacturonan II, which is known to bind heavy metals and not essential mineral cations.15 It also has been shown to decrease total body burden of mercury after prolonged use.16
Recent clinical data indicate that MCP can play an important therapeutic role as a selective chelator of heavy metals.15-17 As heavy metal load may also be a complicating concern in cancer patients, the use of MCP for the dual indications of lessening heavy metal load and halting cancer progression may be an important clinical application.17 Heavy metals, in conjunction with the abundant presence of environmental toxins and xenoestrogens, constitute a dangerous insult to the body through DNA damage, hormonal modulation, immune suppression, oxidative stress, and hyper- infl ammation. They are of particular concern in various cancers, including prostate cancer and breast cancer. The idea that long- term administration of a gentle chelator such as MCP could effec- tively reduce the body burden of heavy metals has been suggested both in case studies with MCP and in pilot clinical trials.15-17 The ability of MCP to remove heavy metals and environmental toxins on an ongoing basis may be of significant clinical benefit.
In one pilot clinical trial, MCP was given to patients and found to increase urinary excretion of heavy metals such as lead, mercury, cadmium, and arsenic. The participants were given 15 g of MCP daily for 5 days and then 20 g on day 6.15 Baseline readings of 24-hour urine samples were compared against 24-hour urine samples on day 1 and day 6. The study found that MCP’s gentle nature allowed for safe chelation with no side effects.15 In an early study by Gralak et al18 the infl uence of citrus pectin on calcium, magnesium, iron, zinc, manganese, and copper absorption was studied in rats. Other soluble and insoluble fiber sources increased fecal excretion and decreased the absorption of calcium, magne- sium, iron, manganese, zinc, and copper, but this was not the case for citrus pectin. Therefore, MCP is a selective chelator, capable of increasing the urinary excretion of heavy metals but not reducing absorption of other essential minerals.
In another pilot clinical trial, MCP was tested for its long- term effects on reducing total mercury body burden in 5 subjects. Baseline total body mercury body burden of the participants was estimated using a 2,3-Dimercapto-1-propanesulfonic acid (DMPS)challenge of 250 mg intravenously followed by 6 hours of urine col- lection. MCP was then administered at a dosage of 15 g/day. Total mercury body burden was then measured again after approxi- mately 4 to 10 months and found to be significantly decreased in all subjects from a mean average of 52 µg/g creatinine to 16 µg/g creatinine. A 69% drop in mercury levels (P=.03) was found for the participants of the study.16
MCP has produced encouraging results in 2 adult human clinical trials in increasing excretion and reducing body burden of toxic metals. This pilot clinical trial evaluates MCP’s effectiveness in reducing lead toxicity in children. The study population is unique because of the public health crisis regarding lead toxicity in urban/industrialized areas in China. In this article we present the results of the reduction of lead in blood serum and the increase in urine excretion in hospitalized children 5 to 12 years of age when MCP is administered as the sole chelating agent or therapy.
MATERIALS AND METHODS
The study was performed at the Children’s Hospital of Zhejiang University, Hangzhou, China. Seven patients were recruit- ed for this study. All patients met the qualification standard of blood lead concentration >20 µg/dL with no chelation and/or detoxification treatments 3 months prior to the study. A parent or guardian of each child provided signed informed consent for partic- ipation in this open-labeled pilot clinical hospital-supervised study.
Patients received 15 g of MCP (PectaSol) daily, divided into three 5-g doses. No other chelation or detoxifying agent was used for 3 months prior to commencement of the study and during the study.
Blood lead concentration and 24-hour-period total urine lead excretion were measured by graphite furnace atomic absorption spectroscopy (GFAAS) at day 0, prior to commencement of MCP consumption and weekly until the patient’s discharge from the hospital. Two patients were released after 2 weeks, 3 after 3 weeks, and 2 after 4 weeks when their blood lead level dropped below the criterion of 20 µg/dL.
Patients were evaluated during the course of hospital treat- ment for subjective symptoms including a worsening or improve- ment of presenting symptoms, development of any new symptoms including potential adverse effects of the MCP, such as headache, diarrhea, reflux or other gastrointestinal disturbance, palpitations, or fl uid retention. General blood analyses were taken and moni- tored for adverse conditions; the results are not included in this report because no abnormal results occurred. The children in this study were not given any special diet due to their condition.
Monosaccharide analysis of MCP was performed following methanolysis using High Performance Anion-Exchange Chromatography and Pulsed Amperometric Detection (HPAEC- PAD). A Dionex DX-500 system (Dionex Corp, Sunnyvale, California)was used, which included a CarboPac PA-20 column operated at 0.5 mL/min. Neutral and acidic monosaccharides were separated in a single run using a mobile phase that was 14 mM sodium hydroxide three 5-g doses. No other chelation or detoxifying agent was used for 3 months prior to commencement of the study and during the study.
Blood lead concentration and 24-hour-period total urine lead excretion were measured by graphite furnace atomic absorption spectroscopy (GFAAS) at day 0, prior to commencement of MCP consumption and weekly until the patient’s discharge from the hospital. Two patients were released after 2 weeks, 3 after 3 weeks, and 2 after 4 weeks when their blood lead level dropped below the criterion of 20 µg/dL.
Patients were evaluated during the course of hospital treat- ment for subjective symptoms including a worsening or improve- ment of presenting symptoms, development of any new symptoms including potential adverse effects of the MCP, such as headache, diarrhea, reflux or other gastrointestinal disturbance, palpitations, or fl uid retention. General blood analyses were taken and moni- tored for adverse conditions; the results are not included in this report because no abnormal results occurred. The children in this study were not given any special diet due to their condition.
Monosaccharide analysis of MCP was performed following methanolysis using High Performance Anion-Exchange Chromatography and Pulsed Amperometric Detection (HPAEC- PAD). A Dionex DX-500 system (Dionex Corp, Sunnyvale, California)was used, which included a CarboPac PA-20 column operated at 0.5 mL/min. Neutral and acidic monosaccharides were separated in a single run using a mobile phase that was 14 mM sodium hydroxide (NaOH) isocratic for 13 minutes, then a 0-120 mM sodium acetate
(CH3COONa) gradient in 100 mM NaOH for 30 minutes. The
mobile phase returned to 14 mM NaOH for 40 minutes prior to the next injection. Other conditions were reported previously.19
RESULTS
Table 1 summarizes the blood lead concentration before and after treatment with MCP. All 7 subjects had a significant decrease in their blood levels of lead. Figure 1 shows the change in blood lead concentration from day 0 and the peak excretion measured during treatment. A statistical analysis using paired sample for means was highly significant (P=.0016). Table 2 summarizes 24-hour total urinary lead excretion of the patients prior to treat- ment and at the peak excretion. Figure 2 shows the change in 24-hour urinary excretion from day 0 and the peak excretion mea- sured during treatment. A statistical analysis using paired sample for means was highly significant (P=.0007). No adverse effects were reported. Three additional children under the age of 5 (between 2.5 and 3.5 years old) were treated with MCP but not included in this study because they were outside of the age criterion. They had similar results and no reported adverse effects. Figure 3 shows the average percent change in the lead levels in the blood and 24-hour urine collection for the subject group.
Figure 4 shows the monosaccharide analysis chromatogram, which demonstrates that MCP consists of 88.2% galacturonic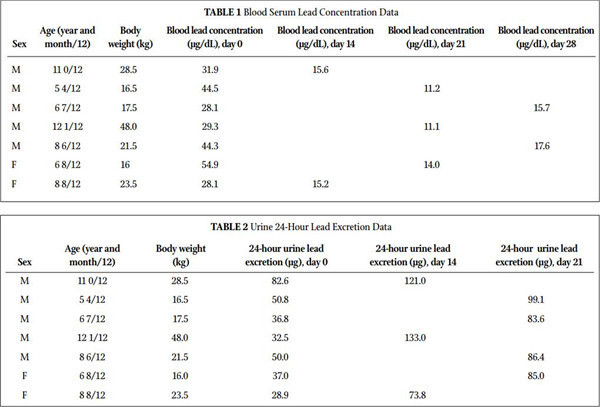
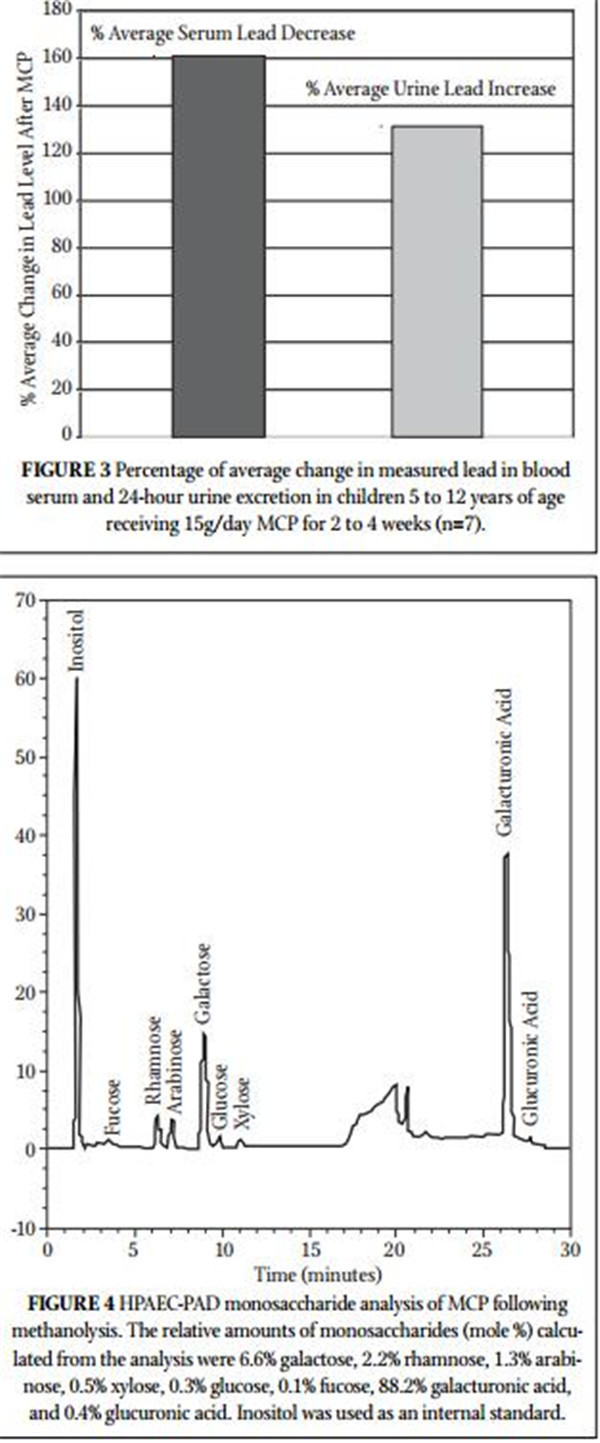
acid. The other MCP monosaccharides are consistent with the reduced molecular weight, debranched, low degree of esterifi ca- tion (3.8%), low rhamnogalacturonan II (10%) pectin structure reported previously.15
DISCUSSION
Lead toxicity in general and in children in particular is a signifi cant global health issue that can cause health problems of varying degrees in multiple systems. This clinical study was undertaken to evaluate the effectiveness of MCP in reducing lead in children hospitalized with measurable lead toxicity. All subjects tested had a signifi cant increase in urinary excretion of lead followed by a signifi cant decrease of blood levels. No side effects were reported. The results clearly demonstrate the effec- tiveness and safety of MCP as a chelator of lead.
Unfortunately, these children return to an environment that chronically exposes their developing bodies to further toxicity and the resulting lifelong damage that comes from lead exposure during the critical developmental years. Lead exposure poses an ongoing health risk for millions of children worldwide. When addressing this issue, we need to remove existing lead toxicity while preventing absorption of lead due to ongoing exposure, and a safe ongoing lead removal agent can work toward this end. Currently, the main agent used for removal of lead toxicity is ethylenediaminetetraacetic acid
(EDTA), which has to be administered intravenously at a high cost and with potential side effects. EDTA, as well as other agents, such as meso-2,3-dimercaptosuccinic acid (DMSA) and DMPS, can deplete the body of essential minerals and redistribute heavy metals with the possibility of increasing their concentration in vital organs like the brain. These agents are therefore not suitable for ongoing use. There is a great need for selective chelating agents that are safe and can be used on an ongoing basis. The long-term use of MCP without apparent side effects has been reported in adults with pros- tate cancer (for 12 months), in the reduction of total body burden of mercury in adults (for 10 months), and in a case report study that used MCP (for 12 months).12,16,17
As a systemic chelator, MCP has the ability to reduce total body burden of heavy metals without side effects. The low degree of esterification homogalacturonan and rhamnogalacturonan II struc- ture of MCP is rich in free carboxyl groups, ideal for the chelation of cations such as lead.20,21 We believe that the rhamnoglacturonan II in MCP confers the selectivity necessary to chelate heavy metals and not the essential mineral cations.15 Apparently, the low molecular weight and rhamnoglacturonan structure allow for gastrointestinal uptake of MCP followed by direct heavy metal binding in the blood- stream and subsequent urinary excretion.15 We have demonstrated systemic chelation of heavy metals by MCP in a clinical study with healthy individuals, those with other chronic ailments and demon- strated heavy metal burden,15,17 and now in this hospital-supervised study in children suffering from chronic lead exposure. The chelat- ing effects of MCP may be enhanced or achieved at lower dosages by combining MCP with modified alginates.17 The results presented here show MCP as an effective chelator, but additional clinical stud- ies are justified to confirm our findings and to optimize the use of MCP on its own or in combination with modified alginates for broad use on an ongoing basis and specifically in children who are chronically exposed to environmental toxins.
Acknowledgments
The authors thank Madhav Yadav and Andre White for technical assistance.
REFERENCES
1. Moore MR, McIntosh MJ, Bushnell IW. The neurotoxicology of lead. Neurotoxicology.
1986;7(2):541-556.
2. Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and
children’s intellectual function: an international pooled analysis. Environ Health Perspect.
2005;113(7):894-899.
3. Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167-182.
4. Rosen JF. Adverse health effects of lead at low exposure levels: trends in the management
of childhood lead poisoning. Toxicology. 1995;97(1-3):11-17.
5. Mendelsohn AL, Dreyer BP, Fierman AH, et al. Low-level lead exposure and cognitive
development in early childhood. J Dev Behav Pediatr. 1999;20(6):425-431.
6. Shen X, Rosen JF, Guo D, Wu S. Childhood lead poisoning in China. Sci Total Environ.
1996;181(2):101-109.
7. Shen X, Wu S, Yan C. Impacts of low-level lead exposure on development of children:
recent studies in China. Clin Chim Acta. 2001;313(1-2):217-220.8. Ye X, Wong O. Lead exposure, lead poisoning, and lead regulatory standards in China,
1990-2005. Regul Toxicol Pharmacol. 2006;46(2):157-162.
9. Modified citrus pectin monograph. Altern Med Rev. 2000;5(6):573-575.
10. Pienta KJ, Naik H, Akhtar A, et al. Inhibition of spontaneous metastasis in a rat prostate
cancer model by oral administration of modified citrus pectin. J Natl Cancer Inst. 1995;87(5):348-353.
11. Hayashi A, Gillen AC, Lott JR. Effects of daily oral administration of quercetin chalcone
and modified citrus pectin on implanted colon-25 tumor growth in Balb-c mice. Altern Med Rev. 2000;5(6):546-552.
12. Guess BW, Scholz MC, Strum SB, Lam RY, Johnson HJ, Jennrich RI. Modified citrus pec-
tin (MCP) increases the prostate-specific antigen doubling time in men with prostate can- cer: a phase II pilot study. Prostate Cancer Prostatic Dis. 2003;6(4):301-304.
13. Hexeberg S, Hexeberg E, Willumsen N, Berge RK. A study on lipid metabolism in heart
and liver of cholesterol- and pectin-fed rats. Br J Nutr. 1994;71(2):181-192.
14. Zhu HG, Zollner TM, Klein-Franke A, Anderer FA. Enhancement of MHC-unrestricted
cytotoxic activity of human CD56+ CD3- natural killer (NK) cells and CD3+ T cells by rhamnogalacturonan: target cell specificity and activity against NK-insensitive targets. J Cancer Res Clin Oncol. 1994;120(7):383-388.
15. Eliaz I, Hotchkiss AT, Fishman ML, Rode D. The effect of modified citrus pectin on uri-
nary excretion of toxic elements. Phytother Res. 2006;20(10):859-864.
16. Eliaz I, Gaurdino J, Hughes K. The health benefits of modified citrus pectin. In: Patil BS,
Turner ND, Miller EG, Brodbelt JS, eds. Potential Health Benefi ts of Citrus. ACS Symposium Series 936. Oxford, UK: Oxford University Press; 2006:199-210.
17. Eliaz I, Weil E, Wilk B. Integrative medicine and the role of modified citrus pectin/alg-
inates in heavy metal chelation and detoxification—five case reports. Forsch Komplement Med (2006). 2007;14(6):358-364.
18. Gralak MA. Leontowicz M, Morawiec M, Bartnikowska E, Kulasek GW. Comparison of
the influence of dietary fibre sources with different proportions of soluble and insoluble fibre on Ca, Mg, Fe, Zn, Mn and Cu apparent absorption in rats. Arch Tiernahr. 1996;49(4):293-299.
19. Manderson K, Pinart M, Tuohy KM, et al. In vitro determination of prebiotic properties
of oligosaccharides derived from orange juice manufacturing by-product stream. Appl Environ Microbial. 2005;71(12):8383-8389.
20. Kohn R. Binding of toxic cations to pectin, its oligomeric fragments and plant tissues.
Carbohydr Polym. 1982;2(4):273-275.
21. O’Neill MA, Warrenfeltz D, Kates K, et al. Rhamnogalacturonan-II, a pectic polysaccha-
ride in the walls of growing plant cell, forms a dimer that is covalently cross-linked by borate ester. In vitro conditions for the formation and hydrolysis of the dimer. J Biol Chem. 1996;27(37)1;22923-22930.- previous:暂无
next:Formation and characterization - back

